Influence of various factors on plasticity and resistance to deformation. Influence of pressure treatment on the structure and properties of the metal
On fig. 2.9 shows graphs of the effect of cold deformation on ductility S, tensile strength a b and hardness HB of mild steel. It can be seen from the graphs that already at a deformation equal to 20%, a decrease in the plasticity of the metal by a factor of 3, an increase in hardness and strength by about 1.3 ... 1.4 times is observed. Therefore, in the cold state, it is impossible to obtain forgings of complex shape from this steel, since the metal will be destroyed during deformation due to low ductility.
To increase the malleability of the processed metals are heated. With an increase in temperature, plasticity increases and the resistance of metals to deformation decreases. As an example, consider the effect of temperature on ductility 5 and tensile strength a in steel with a carbon content of 0.42% (Fig. 2.10). With an increase in the deformation temperature from 0 to 300 °C, the resistance to deformation increases slightly, and then drops from 760 to 10 MN/m 2 at 1200 °C, i.e., it decreases by almost 76 times. The ductility of this steel, on the contrary, with an increase in temperature from 0 to 300 ° C, first decreases, then sharply increases to a temperature of 800 ° C, then drops slightly, and again with a further increase in temperature. increases. The phenomenon of reduced plasticity at 300 °C is called blue brittleness, and at 800 °C it is called red brittleness. Blue brittleness is explained by the precipitation of the smallest particles of carbides along the sliding planes, which increase the resistance to deformation and reduce ductility. Red brittleness appears due to the formation in the metal of a multiphase system with reduced plasticity. This condition is characteristic of incomplete hot working. At temperatures of blue brittleness and red brittleness, it is especially undesirable to deform steel, since during forging cracks may form in the workpiece and, as a result, product defects.
Various metals and alloys are treated by pressure in a well-defined temperature range AT \u003d T b ~ T l, where T in and T n are the upper and lower temperature limits for metal pressure treatment, respectively.
Deformation of the metal at a temperature below T n due to a decrease in ductility can lead to its destruction. Heating the metal above the temperature T in leads to defects in the structure of the metal, a decrease in its mechanical properties and ductility. The temperature ranges of pressure treatment for different metals are different, but they have in common that metals have the greatest plasticity at temperatures exceeding the recrystallization temperature.
Influence of degree and rate of deformation. The degree and rate of deformation have a complex effect on the ductility and resistance of the metal to deformation. Moreover, this influence depends both on their values and on the state in which the metal is deformed - hot or cold.
The degree and rate of deformation simultaneously exert both strengthening and softening effects on the metal. So, with an increase in the degree of deformation, on the one hand, the work hardening of the metal increases, and, consequently, its resistance to deformation also increases. But, on the other hand, an increase in the degree of deformation, intensifying the process of recrystallization, leads to a softening of the metal and a decrease in its resistance to deformation. As for the strain rate, with its increase, the time of the recrystallization process decreases and, consequently, the hardening increases. However, with an increase in the strain rate, the amount of heat released in the metal at the moment of deformation increases, which does not have time to dissipate in environment and causes additional heating of the metal. An increase in temperature is accompanied by a decrease in the resistance of the metal to deformation.
In most cases, manual forging metal is deformed in a heated state and an increase in the degree and rate of deformation leads to a decrease in ductility and an increase in resistance to deformation.
Influence of the stress state scheme. The stress state pattern has a significant effect on ductility, deformation resistance, and total forming force.
The higher the tensile stresses in the deformable metal, the more its ductility decreases and the more likely it is that cracks will appear in it. Therefore, one should strive to process the metal in such a way that compressive stresses arise in it and there are no tensile ones.
So, the metal has the lowest plasticity under deformation conditions according to the linear tension scheme (see Fig. 2.6, / and 2.7, a) and the highest - according to the all-round uneven compression scheme (see Fig. 2.6, iii and 2.11, a). It has been experimentally established that alloys that are nonplastic under conditions of uniaxial tension are well deformed under conditions of uniform nonuniform compression. Cast iron, for example, during tension or open upsetting (see Fig. 2.5) practically does not deform, while it can be subjected to significant deformations by extrusion with a force P and back pressure P p p according to the scheme shown in Fig. 2.11, a.

Knowledge of stress state schemes is of great practical value. When forging high-alloy steels on flat dies (see Fig. 2.5), cracks may appear on the barrel-shaped surface of the workpiece. This is explained by the fact that in this zone the stress state of the metal is characterized by the presence of tensile stresses o 3 . If this workpiece is upset in a mandrel (Fig. 2.11, b) or forged in cut-out dies (Fig. 2.11, c), then the metal stress state scheme will correspond to the all-round compression scheme and, thus, crack formation can be avoided.
In modern forging and stamping production, blanks of parts from some heat-resistant alloys are obtained only by extrusion, since with other methods (upsetting, bending, open stamping), destruction of the alloy is observed.
1. Chemical composition
Pure metals have the highest ductility, the lowest - chemical compounds(greater resistance to dislocation movement).
Alloy additives Cr, Ni, W, Co, Mo - increase plasticity; C, Si - reduce ductility.
2. Micro-, macrostructure
With a decrease in grain size, plasticity increases (superplasticity). Heterogeneity of grains reduces plasticity.
3. Phase composition
The greatest plasticity has a metal of a homogeneous structure. different phases, which have incoherent lattices, hinder the movement of dislocations and reduce plasticity.
In addition, they deform differently, which contributes to the formation of cracks.
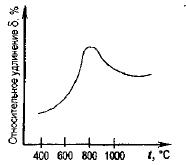
The decrease in plasticity at temperatures above 800°C is associated with the formation of the second phase - residual ferrite. The increase in plasticity at temperatures above 1000°C indicates a sharp decrease in the resistance of the metal to deformation.
4. Strain rate
It is necessary to distinguish between the speed of movement of the tool or the speed of deformation (V, m / s) and the speed of deformation - a change in the degree of deformation per unit of time (u or ε, s-1),
where L is the base length of the sample subjected to tension; Δl - absolute elongation of the sample Δl=l-L; t - time; V is the speed of the tool; H, h - body height, respectively, before and after deformation; Ah - absolute reduction Δh = H-h; R is the radius of the working rolling rolls.
As the strain rate increases, plasticity decreases., because it can't move the right number dislocations.
The increase in plasticity at high strain rates is explained by an increase in the temperature of the metal.
5. Environment. Some surfactants increase the plasticity of the metal (oleic acid) - facilitate plastic shear, others - contribute to brittle fracture (kerosene).
Thus, due attention must be paid to lubricants.

Rolling in vacuum or medium inert gases rare earth elements (Nb, Mo, Te) does not allow the formation of an oxide film, which is very fragile. When rolling in a vacuum, the gas diffuses outward and the metal becomes ductile. Shops with a protective atmosphere have been built in the USA. In the city of Chirchik (Tajikistan), a rolling mill operates at a metallurgical plant with sealed roll assemblies in which a vacuum is created.
6. Fractionality of deformation
An increase in the fragmentation of deformation leads to an increase in the plasticity of alloyed steel grades.

Rolling on a planetary mill, due to the high degree of deformation, allows you to get 98% of the degree of deformation. Fractional deformation helps to reduce the non-uniformity of the metal structure, facilitates the rotation of the grains. When reloading, there is a decrease in residual stresses between the grain and the boundary zones,
7. Mechanical scheme of deformation
The most favorable scheme of plastic deformation is the scheme of three-sided non-uniform compression. Other things being equal, a decrease in tensile stress has a beneficial effect on the plastic properties of the metal.
In the transition from deformation according to the scheme of uniaxial tension to deformation according to the scheme of three-sided compression, it is theoretically possible to increase the plasticity of the metal by 2.5 times.
In Karman's classical experiments on pressing marble and sandstone, a value of 68% of the degree of deformation of marble was obtained without destruction when treated with high hydrostatic pressure.
hydrostatic pressure
![]()
where σ1, σ2, σ3 are the principal compressive stresses.
Plastic deformation occurs due to the difference in principal stresses σ1 ~ σ3 = σt.
When rolling brittle cast alloys, to reduce tensile stresses on the edges, the so-called “jacket” is used (before rolling, the workpiece is wrapped in a shell of highly ductile metal). In this case, tensile stresses arise in the shell, and the deformable metal experiences compressive stresses that prevent cracking.

A promising direction is the use of hydroextrusion - the creation of a comprehensive non-uniform compressive pressure in a deformable metal due to a liquid (to be discussed later).
In real processes, there is always deformation unevenness (between grains, between individual local areas), which causes deformation unevenness.
8. Scale factor
The larger the volume of the body, the lower its plastic properties, all other things being equal, should be taken into account when developing MMD processes and when designing equipment.

05.04.2019
Grapes are berries short term storage. Even in the refrigerator, it very quickly becomes lethargic, loses its normal appearance. You can, of course, freeze it in ...
05.04.2019
An experienced specialist of a company that provides services for installation, repair and...
05.04.2019
A gas boiler is an equipment, with its help, thermal energy is obtained, which is required for normal heating of a room. These units often...
05.04.2019
On the territory of the Tashkent metallurgical enterprise, they began to bring the main technological equipment. The MetProm Group of Companies acted as a supplier in...
05.04.2019
From the first day of the emergence of secured loans, borrowers have the opportunity to take significant amounts of money on best conditions than in the case of registration ...
05.04.2019
Today, any company operating in the chemical industry uses special equipment in carrying out various procedures, where various ...
05.04.2019
A well-known corporation from Canada, First Quantum Minerals, which in the winter of this year transferred the mine for the extraction of copper raw materials Cobre Panama in the territory of...
05.04.2019
VVGNG-LS is a power cable that provides electrical power to stationary (as part of various buildings), as well as mobile (in construction site conditions)...
Plastic - the ability of the metal to perceive the residual deformation without destruction.
Sometimes, high ductility and low resistance to deformation are mistakenly identified. Plasticity and resistance to deformation are different characteristics of solids that do not depend on each other.
The ability to plastically change shape is inherent in all solids, but in some of them it is negligible and manifests itself only during deformation under special conditions.
Factors affecting plasticity:
1. The nature of the substance: pure metals have good plasticity, and impurities that form solid solutions with the metal reduce plasticity less than those that do not dissolve in it. Especially noticeably reduce the plasticity of impurities that precipitate during crystallization along the grain boundaries;
2. Hardening: due to the phenomenon of self-hardening, which accompanies hardening, the plasticity of the metal decreases;
3.Temperature: an increase in the temperature of the metal leads to an increase in ductility. At very low temperatures the metal becomes brittle. There are temperature intervals that are different for different metals. In carbon steel, a noticeable decrease in ductility is found at temperatures in , called blue brittleness. This phenomenon is explained by the release of the smallest particles of carbides along the slip planes.
With insufficient manganese content in low-carbon steel, a sharp drop in ductility at a temperature of c is called red brittleness. This phenomenon occurs due to the melting of the FeS eutectic located along the grain boundaries.
Leads to a sharp drop in plastic properties burnout - a defect formed as a result of a long exposure of the metal in the zone high temperatures close to the melting temperature, accompanied by oxidation of the grain surface, weakening intergranular bonds. Burnout is an irreparable defect.
A decrease in plasticity is also observed at overheating - a defect formed as a result of holding the metal in the high temperature zone, accompanied by excessive coarsening of grains in the region of phase transformations. Overheating is a removable defect and is solved by subsequent heat treatment;
4. Deformation rate: during hot working of metals, due to the retardation of the recrystallization process from work hardening, an increase in speed reduces plasticity. During cold working, an increase in the strain rate can increase ductility due to the heating of the metal by the released heat;
5. The nature of the stress state: According to the views existing in the theory of metal forming, plastic deformation occurs under the influence of shear stresses, and brittle fracture is caused by normal tensile stresses. The influence of the stress state on plasticity can be estimated from the value of hydrostatic pressure: 
If the hydrostatic pressure increases, then the plasticity increases, if it decreases, then the plasticity decreases. Experience shows that by changing the stress state, everything is possible solid bodies considered ductile or brittle, therefore plasticity is considered not a property, but a state of matter;
^Factors affecting the ductility of the metal
Plasticity depends on the nature of the substance (its chemical composition and structural structure), temperature, strain rate, hardening degree, and stress conditions at the moment of deformation.
^ Influence of natural properties of metal. Plasticity is directly dependent on the chemical composition of the material. With increasing carbon content in steel, ductility decreases. The elements that make up the alloy as impurities have a great influence. Tin, antimony, lead, sulfur do not dissolve in the metal and, located along the grain boundaries, weaken the bonds between them. The melting point of these elements is low; when heated for hot deformation, they melt, which leads to a loss of ductility. Substitutional impurities reduce plasticity less than interstitial impurities.
Plasticity depends on the structural state of the metal, especially during hot deformation. The heterogeneity of the microstructure reduces plasticity. Single-phase alloys, ceteris paribus, are always more ductile than two-phase ones. The phases are not the same mechanical properties, and the deformation is uneven. Fine-grained metals are more ductile than coarse-grained ones. The metal of ingots is less ductile than the metal of a rolled or forged billet, since the cast structure has a sharp heterogeneity of grains, inclusions, and other defects.
^ Temperature effect . At very low temperatures, close to absolute zero, all metals are brittle. Low ductility must be taken into account in the manufacture of structures operating at low temperatures.
With an increase in temperature, the ductility of low-carbon and medium-carbon steels increases. This is explained by the fact that grain boundary violations are corrected. But the plasticity increase is not monotonous. In the intervals of certain temperatures, a "failure" of plasticity is observed. So for pure iron, brittleness is found at a temperature of 900-1000 ° C. This is due to phase transformations in the metal. The decrease in plasticity at a temperature of 300-400 ° C is called blue brittleness, at a temperature of 850-1000 about C - red brittleness.
High-alloy steels have greater cold ductility . For ball-bearing steels, ductility is practically independent of temperature. Individual alloys may have a range of increased ductility .
When the temperature approaches the melting point, the ductility decreases sharply due to overheating and overburning. Overheating is expressed in the excessive growth of grains of pre-deformed metal. Overheating is corrected by heating to a certain temperature and then rapid cooling. Burnout is an incorrigible marriage. It consists in the oxidation of the boundaries of large grains. In this case, the metal is brittle.
^ Influence of work hardening and strain rate . Hardening reduces the ductility of metals.
The effect of strain rate on plasticity is twofold. During hot working by pressure, an increase in speed leads to a decrease in plasticity, because. hardening is ahead of recrystallization. During cold working, an increase in the strain rate most often increases ductility due to heating of the metal.
^ Influence of the nature of the stress state. The nature of the stress state has big influence for plasticity. An increase in the role of compressive stresses in general scheme stressed state increases plasticity. Under conditions of pronounced all-round compression, it is possible to deform even very brittle materials. The scheme of all-round compression is the most favorable for the manifestation of plastic properties, since intergranular deformation is hindered in this case and all deformation proceeds due to intragranular deformation. An increase in the role of tensile stresses leads to a decrease in plasticity. Under conditions of all-round tension with a small difference in principal stresses, when shear stresses are small for the onset of plastic deformation, even the most ductile materials are brittle fracture.
Plasticity can be assessed using . If a  increases, the plasticity also increases, and vice versa. Experience shows that by changing the state of stress, it is possible to make all solid bodies ductile or brittle. So plasticity is considered not a property, but a special state of matter.
increases, the plasticity also increases, and vice versa. Experience shows that by changing the state of stress, it is possible to make all solid bodies ductile or brittle. So plasticity is considered not a property, but a special state of matter.
^
Plasticity condition
Plasticity condition for linear stress state
The plasticity condition is the condition for the transition of elastic deformation into plastic, i.e. it defines the inflection point in the tension-compression diagram.
In a linear stress state, for example, when a sample is stretched, plastic deformation begins when the normal stress reaches the yield point. That is, for a linear stress state, the plasticity condition has the form:  .
.
Note: in the process of deformation  changes. Therefore, in the theory of plasticity, instead of the concept of "yield stress", the concept of "resistance to deformation" is used, i.e. the specific force that brings the sample into a plastic state in the process of uniform linear tension at a given temperature, a given rate and degree of deformation.
changes. Therefore, in the theory of plasticity, instead of the concept of "yield stress", the concept of "resistance to deformation" is used, i.e. the specific force that brings the sample into a plastic state in the process of uniform linear tension at a given temperature, a given rate and degree of deformation.
In the volume stressed state, there must also be a certain ratio between the resistance to deformation  and principal normal stresses for the onset of plastic deformation.
and principal normal stresses for the onset of plastic deformation.
^
The condition of constancy of the maximum shear stress (the Saint-Venant plasticity condition)
On the basis of experimental data, Tresca found that for the onset of plastic deformation, the maximum shear stress must reach a certain, constant value for a given metal. Saint-Venant derived the condition of plasticity on the basis of these experiments. He found that plastic deformation occurs when the maximum shear stress reaches a value half yield strength, i.e.  . But
. But  . From here we get
. From here we get  .
.
Thus, the plasticity condition Saint Venant looks like:
Plastic deformation occurs when the maximum difference between the main normal stresses reaches the value of resistance to deformation, i.e. 
In arbitrary axes, the plasticity equation has the form:
An experimental verification of this law showed a discrepancy between theory and practice of 0-16%. This is because the equation does not take into account the influence of the average principal stress  .
.
^
Energy plasticity condition (Huber–Mises–Genka plasticity condition)
According to the Saint-Venant plasticity condition, the transition of a body from an elastic state to a plastic one does not depend on the average stress. M. Huber, Z. Mises and G. Genki proposed a new plasticity condition:
Plastic deformation occurs when the stress intensity reaches a value equal to the yield strength in a linear stress state, i.e. 
After substituting the formula for the stress intensity, we obtain
Or in the main stresses
Considering that in a linear stress state  , we get
, we get  .
.
This plasticity condition is also called the condition of constancy of stress intensity or the condition of constancy of shear stress intensity or the condition of constancy of octahedral stresses.
The Huber-Mises-Genka plasticity condition is called the energy plasticity condition, because it was derived from the energy condition: plastic deformation occurs when potential energy elastic deformation, aimed at changing the shape of the body, will reach certain value regardless of the stress state scheme.
It follows from the plasticity condition that the condition for the transition from elastic to plastic deformation does not depend on the absolute value of the principal stresses, but depends only on their difference. An increase or decrease in the principal stresses by the same value does not change the conditions for the onset of plastic deformation, i.e. the transition from the elastic state to the plastic one does not depend on the spherical tensor, but depends only on the stress deviator.
For further transformations, we introduce a dimensionless quantity - the directing stress tensor:  , we express
, we express  through
through  :
:  and substitute into the plasticity equation:
and substitute into the plasticity equation:
After transformations, we get: 
O  designate
designate  , then the plasticity equation will take the form:
, then the plasticity equation will take the form: 
Coefficient  is called the Lode coefficient after the name of the scientist,
is called the Lode coefficient after the name of the scientist,
Experimentally verified the equation of plasticity.
Insofar as  , the following extreme cases are possible:
, the following extreme cases are possible:
 , then
, then  and
and  ;
;
 , then
, then  and ;
and ;
 , then
, then  and
and  ;
;
those. the Lode coefficient takes values from 1 to 1.15. In the case when , the plasticity equation takes the form  , i.e. coincides with the Saint-Venant plasticity condition. In case when
, i.e. coincides with the Saint-Venant plasticity condition. In case when  , the discrepancy between the plasticity conditions is the maximum value (about 16%).
, the discrepancy between the plasticity conditions is the maximum value (about 16%).
