The value of the specific heat of vaporization of hydrochloric acid. Boiling. Specific heat of vaporization
The process of changing a substance from a liquid state to a gaseous state is called vaporization. Vaporization can be carried out in the form of two processes: i.
Boiling
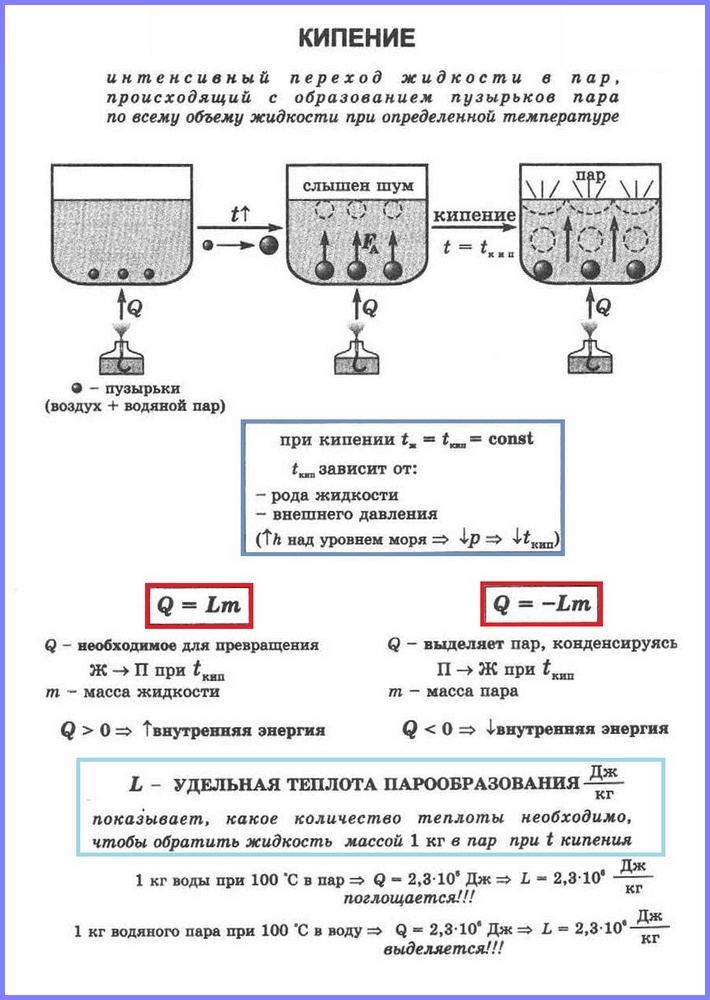
The second process of vaporization is boiling. You can watch this process with simple experience heating water in a glass flask. When water is heated, bubbles appear in it after a while, which contain air and saturated water vapor, which is formed during the evaporation of water inside the bubbles. When the temperature rises, the pressure inside the bubbles increases, and under the action of the buoyancy force, they rise up. However, since the temperature of the upper layers of water is lower than the lower ones, the vapor in the bubbles begins to condense and they shrink. When the water warms up throughout the volume, the bubbles with steam rise to the surface, burst, and the steam comes out. Water is boiling. This occurs at a temperature at which the pressure saturated steam bubbles is equal to atmospheric pressure.
The process of vaporization occurring in the entire volume of a liquid at a certain temperature is called. The temperature at which a liquid boils is called boiling point.
This temperature depends on atmospheric pressure. As atmospheric pressure rises, the boiling point rises.
Experience shows that during the boiling process the temperature of the liquid does not change, despite the fact that energy comes from outside. The transition of a liquid to a gaseous state at the boiling point is associated with an increase in the distance between the molecules and, accordingly, with overcoming the attraction between them. The energy supplied to the fluid is expended to do the work of overcoming the forces of attraction. This happens until all the liquid turns into vapor. Since liquid and vapor have the same temperature during boiling, the average kinetic energy molecules does not change, only their potential energy increases.
The figure shows a graph of water temperature versus time during its heating from room temperature to boiling (AB), boiling (BC), steam heating (CD), steam cooling (DE), condensation (EF) and subsequent cooling (FG) .
Specific heat of vaporization
For the transformation of different substances from a liquid state into a gaseous state, different energy is required, this energy is characterized by a value called the specific heat of vaporization.
Specific heat vaporization (L) is a value equal to the ratio of the amount of heat that must be imparted to a substance with a mass of 1 kg to transform it from a liquid state into a gaseous state at the boiling point.
The unit of specific heat of vaporization is [ L] = J/kg.
To calculate the amount of heat Q, which must be imparted to a substance with a mass mn for its transformation from a liquid state to a gaseous one, it is necessary to have the specific heat of vaporization ( L) times the mass of the substance: Q = Lm.
When steam condenses, a certain amount of heat is released, and its value is equal to the value of the amount of heat that must be spent to turn the liquid into steam at the same temperature.
In order to maintain the boiling of water (or other liquid), it is necessary to continuously supply heat to it, for example, to heat it with a burner. In this case, the temperature of the water and the vessel does not rise, but a certain amount of steam is formed for each unit of time. From this follows the conclusion that the transformation of water into vapor requires an influx of heat, just as it takes place during the transformation of a crystal (ice) into a liquid (§ 269). The amount of heat required to convert a unit mass of a liquid into vapor of the same temperature is called the specific heat of vaporization of a given liquid. It is expressed in joules per kilogram.
It is easy to see that the same amount of heat must be released when a vapor condenses into a liquid. Indeed, let us lower a tube connected to a boiler into a glass of water (Fig. 488). Some time after the start of heating, air bubbles will begin to come out of the end of the tube dipped into the water. This air slightly raises the temperature of the water. Then the water in the boiler boils, after which we will see that the bubbles coming out of the end of the tube no longer rise up, but quickly decrease and sharp sound disappear. These are bubbles of steam condensing into water. As soon as steam comes out of the boiler instead of air, the water will begin to heat up quickly. As specific heat steam is approximately the same as air, then from this observation it follows that such a rapid heating of water occurs precisely due to the condensation of steam.

Rice. 488. While air is coming out of the boiler, the thermometer shows almost the same temperature. When steam comes out instead of air and starts to condense in the cup, the thermometer will quickly rise, indicating an increase in temperature.
When a unit mass of vapor condenses into a liquid of the same temperature, an amount of heat is released equal to the specific heat of vaporization. This could be foreseen on the basis of the law of conservation of energy. Indeed, if this were not so, then it would be possible to build a machine in which the liquid first evaporated and then condensed: the difference between the heat of vaporization and the heat of condensation would represent the increment in the total energy of all bodies participating in the process under consideration. And this contradicts the law of conservation of energy.
The specific heat of vaporization can be determined using a calorimeter, similar to how it is done when determining the specific heat of fusion (§ 269). Pour a certain amount of water into the calorimeter and measure its temperature. Then, for some time, we will introduce vapor of the test liquid from the boiler into the water, taking measures to ensure that only steam flows, without droplets of liquid. To do this, steam is passed through a steamer (Fig. 489). After that, we again measure the water temperature in the calorimeter. By weighing the calorimeter, we can judge by the increase in its mass the amount of vapor condensed into a liquid.

Rice. 489. Sukhoparnik - a device for retaining water droplets moving along with steam
Using the law of conservation of energy, we can compose an equation for this process heat balance, which allows to determine the specific heat of vaporization of water. Let the mass of water in the calorimeter (including the water equivalent of the calorimeter) be equal to the mass of steam - , the heat capacity of water - , the initial and final temperatures of water in the calorimeter - and , the boiling point of water - and the specific heat of vaporization - . The heat balance equation has the form
![]() .
.
The results of determining the specific heat of vaporization of some liquids at normal pressure are given in Table. 20. As you can see, this heat is quite large. The high heat of vaporization of water plays an extremely important role in nature, since the processes of vaporization occur in nature on a grandiose scale.
Table 20. Specific heat of vaporization of some liquids
|
Substance |
Substance |
||
|
Ethanol) |
|||
Note that the values of the specific heat of vaporization contained in the table refer to the boiling point at normal pressure. If the liquid boils or simply evaporates at a different temperature, then its specific heat of vaporization is different. As the temperature of a liquid rises, the heat of vaporization always decreases. We will look at the explanation for this later.
295.1. Calculate the amount of heat required to heat 20 g of water to the boiling point and turn 20 g of water into steam at .
295.2. What temperature will be obtained if 3 g of steam is introduced into a glass containing 200 g of water at ? Ignore the heat capacity of the glass.
We all know that water in a kettle boils at 100°C. But have you noticed that the temperature of water does not change during the boiling process? The question is - where does the generated energy go if we constantly keep the container on fire? It goes into converting liquid into steam. Thus, for the transition of water into a gaseous state, a constant supply of heat is required. How much it is needed to convert a kilogram of liquid into steam of the same temperature is determined by a physical quantity called the specific heat of vaporization of water.
The physical meaning of the quantity
Boiling requires energy. Most of it is used to break chemical bonds between atoms and molecules, resulting in the formation of vapor bubbles, and the smaller one goes to expand the vapor, that is, so that the formed bubbles can burst and release it. Since the liquid puts all its energy into the transition to the gaseous state, its "forces" run out. For constant renewal of energy and prolongation of boiling, more and more heat must be brought to the container with liquid. A boiler, gas burner or any other can provide its inflow. heating device. During boiling, the temperature of the liquid does not increase, the process of formation of steam of the same temperature takes place.
Different liquids require different amount heat to convert to steam. Which one - shows the specific heat of vaporization.
You can understand how this value is determined from an example. Take 1 liter of water and bring it to a boil. Then we measure the amount of heat needed to evaporate all the liquid, and we get the value of the specific heat of vaporization for water. For others chemical compounds this indicator will be different.
In physics, the specific heat of vaporization is denoted Latin letter L. It is measured in joules per kilogram (J/kg). It can be derived by dividing the heat expended on evaporation by the mass of the liquid:
This value is very important for production processes based modern technologies. For example, they are guided by it in the production of metals. It turned out that if iron is melted and then condensed, with further hardening, a stronger crystal lattice is formed.
What is equal to
The value of specific heat for various substances (r) was determined during laboratory research. Water at normal atmospheric pressure boils at 100 °C, and the heat of vaporization of water is 2258.2 kJ/kg. This indicator for some other substances is given in the table:
| Substance | boiling point, °C | r, kJ/kg |
|---|---|---|
| Nitrogen | -196 | 198 |
| Helium | -268,94 | 20,6 |
| Hydrogen | -253 | 454 |
| Oxygen | -183 | 213 |
| Carbon | 4350 | 50000 |
| Phosphorus | 280 | 400 |
| Methane | -162 | 510 |
| Pentane | 36 | 360 |
| Iron | 2735 | 6340 |
| Copper | 2590 | 4790 |
| Tin | 2430 | 2450 |
| Lead | 1750 | 8600 |
| Zinc | 907 | 1755 |
| Mercury | 357 | 285 |
| Gold | 2 700 | 1 650 |
| Ethanol | 78 | 840 |
| Methyl alcohol | 65 | 1100 |
| Chloroform | 61 | 279 |
However, this indicator can change under the influence of certain factors:
- Temperature. As it increases, the heat of vaporization decreases and can be zero.
t, °C r, kJ/kg 2500 10 2477 20 2453 50 2380 80 2308 100 2258 200 1940 300 1405 374 115 374,15 - Pressure. As the pressure decreases, the heat of vaporization increases, and vice versa. The boiling point is directly proportional to pressure and can reach a critical value of 374 °C.
p, Pa bp, °C r, kJ/kg 0,0123 10 2477 0,1234 50 2380 1 100 2258 2 120 2202 5 152 2014 10 180 1889 20 112 1638 50 264 1638 100 311 1316 200 366 585 220 373,7 184,8 Critical 221.29 374,15 - - The mass of the substance. The amount of heat involved in the process is directly proportional to the mass of the resulting steam.
The ratio of evaporation and condensation
Physicists have found that the reverse evaporation process - condensation - steam spends exactly the same amount of energy as was spent on its formation. This observation confirms the law of conservation of energy.
Otherwise, it would be possible to create an installation in which the liquid would evaporate and then condense. The difference between the heat required for evaporation and the heat sufficient for condensation would result in the accumulation of energy that could be used for other purposes. In fact, a perpetual motion machine would be created. But this is contrary to physical laws, and therefore impossible.
How is it measured
- The specific heat of vaporization of water is measured experimentally in physical laboratories. For this, calorimeters are used. The procedure is as follows:
- A certain amount of liquid is poured into the calorimeter.
Boiling is an intense vaporization that occurs when a liquid is heated not only from the surface, but also inside it.
Boiling occurs with the absorption of heat.
Most of of the supplied heat is spent on breaking the bonds between the particles of the substance, the rest is spent on the work done during the expansion of the steam.
As a result, the interaction energy between vapor particles becomes greater than between liquid particles, so the internal energy of the vapor is greater than the internal energy of the liquid at the same temperature.
The amount of heat required to transfer liquid to vapor during the boiling process can be calculated using the formula:

where m is the mass of liquid (kg),
L is the specific heat of vaporization.
The specific heat of vaporization shows how much heat is needed to turn 1 kg of a given substance into steam at the boiling point. The unit of specific heat of vaporization in the SI system:
[ L ] = 1 J/kg
As the pressure increases, the boiling point of the liquid rises, and the specific heat of vaporization decreases, and vice versa.

During boiling, the temperature of the liquid does not change.
The boiling point depends on the pressure exerted on the liquid.
Each substance at the same pressure has its own boiling point.
With an increase in atmospheric pressure, boiling begins at more high temperature, vice versa when the pressure decreases.
For example, water boils at 100°C only at normal atmospheric pressure.
WHAT HAPPENS INSIDE THE LIQUID WHEN BOILING?

Boiling is the transition of a liquid into vapor with the continuous formation and growth of vapor bubbles in the liquid, inside which the liquid evaporates. At the beginning of heating, the water is saturated with air and has room temperature. When water is heated, the gas dissolved in it is released at the bottom and walls of the vessel, forming air bubbles. They begin to appear long before boiling. Water evaporates into these bubbles. A bubble filled with steam begins to inflate at a sufficiently high temperature.

Reaching certain sizes it breaks away from the bottom, rises to the surface of the water and bursts. In this case, the vapor leaves the liquid. If the water is not heated enough, then the steam bubble, rising into the cold layers, collapses. The resulting water fluctuations lead to the appearance of a huge number of small air bubbles in the entire volume of water: the so-called "white key".

An air bubble with a volume at the bottom of the vessel acts lifting force:
Fpod \u003d Farchimede - Fgravity
The bubble is pressed to the bottom, since pressure forces do not act on the lower surface. When heated, the bubble expands due to the release of gas into it and breaks away from the bottom when the lifting force is slightly greater than the pressing one. The size of a bubble that can break away from the bottom depends on its shape. The shape of the bubbles at the bottom is determined by the wettability of the vessel bottom.
Wetting inhomogeneity and merging of bubbles at the bottom led to an increase in their size. At large sizes When a bubble rises behind it, voids, gaps and eddies are formed.
When the bubble bursts, all the liquid surrounding it rushes inward, and an annular wave occurs. Closing, she throws up a column of water.

When bursting bubbles collapse in a liquid, shock waves of ultrasonic frequencies propagate, accompanied by audible noise. For initial stages boiling is characterized by the loudest and highest sounds (at the stage of the "white key" the kettle "sings").
(source: virlib.eunnet.net)
TEMPERATURE GRAPH OF CHANGES IN AGGREGATE STATES OF WATER

LOOK AT THE BOOKSHELF!
INTERESTING
Why is there a hole in the lid of the teapot?
To release steam. Without a hole in the lid, steam can slosh water over the kettle's spout.
___
The duration of cooking potatoes, starting from the moment of boiling, does not depend on the power of the heater. The duration is determined by the residence time of the product at the boiling point.
The power of the heater does not affect the boiling point, but only the rate of water evaporation.
Boiling can make water freeze. To do this, it is necessary to pump out air and water vapor from the vessel where the water is located, so that the water boils all the time.
"Pots easily boil over the edge - to bad weather!"
The drop in atmospheric pressure that accompanies worsening weather is the reason why milk "runs away" faster.
___
Very hot boiling water can be obtained at the bottom of deep mines, where the air pressure is much greater than on the surface of the Earth. So at a depth of 300 m, water will boil at 101 ͦ C. With an air pressure of 14 atmospheres, water boils at 200 ͦ C.
Under the bell of the air pump, you can get "boiling water" at 20 ͦ C.
On Mars, we would drink "boiling water" at 45 C.
Salt water boils above 100 ͦ C. ___
In mountainous regions at a considerable height, under reduced atmospheric pressure, water boils at temperatures lower than 100 ͦ Celsius.

Waiting for such a meal to be cooked takes longer.
Pour it cold ... and it will boil!
Normally, water boils at 100 degrees Celsius. Heat the water in the flask on the burner to a boil. Let's turn off the burner. The water stops boiling. We close the flask with a stopper and begin to carefully pour cold water onto the stopper. What is it? The water is boiling again!
..............................under the jet cold water some water in the flask, and with it the water vapor begins to cool.
The vapor volume decreases and the pressure above the water surface changes...
What do you think, in which direction?
... The boiling point of water at reduced pressure is less than 100 degrees, and the water in the flask boils again!
____
When cooking, the pressure inside the pot - "pressure cooker" - is about 200 kPa, and the soup in such a pot will cook much faster.
You can draw water into the syringe up to about half, close it with the same cork and pull the piston sharply. A lot of bubbles will appear in the water, indicating that the process of boiling water has begun (and this is at room temperature!).
___
When a substance passes into a gaseous state, its density decreases by about 1000 times.
___
The first electric kettles had heaters under the bottom. The water did not come into contact with the heater and boiled for a very long time. In 1923, Arthur Large made a discovery: he placed a heater in a special copper tube and placed it inside the kettle. The water boiled quickly.
Self-cooling cans for soft drinks have been developed in the USA. A compartment with a low-boiling liquid is mounted in the jar. If you crush the capsule on a hot day, the liquid will begin to boil rapidly, taking away heat from the contents of the jar, and in 90 seconds the temperature of the drink drops by 20-25 degrees Celsius.
WHY?
Do you think it is possible to hard boil an egg if the water boils at a temperature lower than 100 degrees Celsius?
____
Will water boil in a pot that is floating in another pot of boiling water?
Why? ___
Can you make water boil without heating it?

Boiling, as we have seen, is also evaporation, only it is accompanied by the rapid formation and growth of vapor bubbles. It is obvious that during boiling it is necessary to bring a certain amount of heat to the liquid. This amount of heat goes to the formation of steam. Moreover, different liquids of the same mass require different amounts of heat to turn them into steam at the boiling point.
Experiments have shown that the evaporation of water weighing 1 kg at a temperature of 100 °C requires 2.3 x 10 6 J of energy. For the evaporation of 1 kg of ether taken at a temperature of 35 °C, 0.4 10 6 J of energy is needed.
Therefore, in order for the temperature of the evaporating liquid not to change, a certain amount of heat must be supplied to the liquid.
The physical quantity showing how much heat is needed to turn a liquid of mass 1 kg into vapor without changing the temperature is called the specific heat of vaporization.
The specific heat of vaporization is denoted by the letter L. Its unit is 1 J / kg.
Experiments have established that the specific heat of vaporization of water at 100 °C is 2.3 10 6 J/kg. In other words, it takes 2.3 x 10 6 J of energy to convert 1 kg of water into steam at a temperature of 100 °C. Therefore, at the boiling point, the internal energy of a substance in the vapor state is greater than the internal energy of the same mass of substance in the liquid state.
Table 6
Specific heat of vaporization of certain substances (at the boiling point and normal atmospheric pressure)
In contact with a cold object, water vapor condenses (Fig. 25). In this case, the energy absorbed during the formation of steam is released. Precise experiments show that, when condensed, steam gives off the amount of energy that went into its formation.

Rice. 25. Steam condensation
Consequently, when 1 kg of water vapor is converted at a temperature of 100 °C into water of the same temperature, 2.3 x 10 6 J of energy is released. As can be seen from a comparison with other substances (Table 6), this energy is quite high.
The energy released during the condensation of steam can be used. At large thermal power plants, the steam used in the turbines heats water.
The water heated in this way is used for heating buildings, in baths, laundries and for other domestic needs.
To calculate the amount of heat Q required to convert any mass of liquid taken at the boiling point into vapor, you need to multiply the specific heat of vaporization L by the mass m:
From this formula, it can be determined that
m=Q/L, L=Q/m
The amount of heat released by steam of mass m, condensing at the boiling point, is determined by the same formula.
Example. How much energy is required to turn 2 kg of water at 20°C into steam? Let's write down the condition of the problem and solve it.

Questions
- What is the energy supplied to the liquid during boiling?
- What is the specific heat of vaporization?
- How can one show experimentally that energy is released when steam condenses?
- What is the energy released by 1 kg water vapor during condensation?
- Where in technology is the energy released during the condensation of water vapor used?
Exercise 16
- How should one understand that the specific heat of vaporization of water is 2.3 10 6 J/kg?
- How should one understand that the specific heat of condensation of ammonia is 1.4 10 6 J/kg?
- Which of the substances listed in Table 6, when converted from a liquid state to a vapor, has an increase in internal energy more? Justify the answer.
- How much energy is required to turn 150 g of water into steam at 100°C?
- How much energy must be expended in order to bring water of mass 5 kg, taken at a temperature of 0 ° C, to a boil and evaporate it?
- What amount of energy will be released by water of mass 2 kg when cooled from 100 to 0 °C? What amount of energy will be released if instead of water we take the same amount of steam at 100 °C?
Exercise
- According to table 6, determine which of the substances, when converted from a liquid state to a vapor, the internal energy increases more strongly. Justify the answer.
- Prepare a report on one of the topics (optional).
- How dew, frost, rain and snow are formed.
- The water cycle in nature.
- Metal casting.
